MassNeb Nebulizer
Good Practice and Maintenance Guidelines
Introduction
The MassNeb nebulizer is inert and provide exceptional performance characteristics, robustness and durability. lt is suitable for use with both organic solvents and aqueous solutions, including HF digests and samples with high levels of Total Dissolved Solids (TDS).
As a high precision nebulizer with tight dimensional tolerances,
the MassNeb nebulizer requires careful handling to ensure optimum performance, especially with extended use. This guide provides recommendations for handling and routine maintenance.
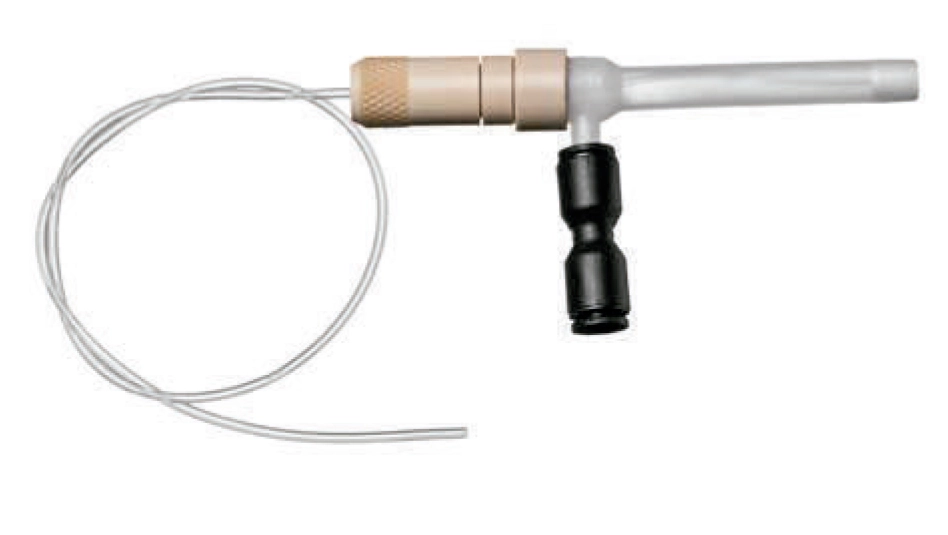
PREVENTING BLOCKAGE OF THE NEBULIZER
Solid particles in the nebulizer gas supply can be potential sources of nebulizer blockage. Removal of dust, fibers and other particles from the vicinity of the nebulizer is essential to minimize the potential for blockage. Follow these guidelines to minimize potential blockage:
1.1 Keep the immediate area clean during nebulizer installation.
1.2 Purge the gas lines to remove any foreign material before connecting the nebulizer gas supply.
This can prevent foreign material from the tube-cutting and installation process (shavings, dust, etc.) from blocking the nebulizer.
1.3 Minimize removal and replacement of the quick-connector from the nebulizer gas inlet. Only change or remove the quick-connector for the nebulizer gas supply when absolutely necessary. Fine plastic shavings or particles may be produced when removing or replacing this connector. These may block the nebulizer. Repeated removal and reconnection can also wear the inside surfaces of the quick-connector, inducing leaks in the nebulizer gas supply. This may result in erratic nebulizer performance and reduced sensitivity. If you observe erratic performance or poor precision, check the quick-connector for leaks and replace if necessary.
Minimize liquid flow through the nebulizer with the nebulizer gas flow off
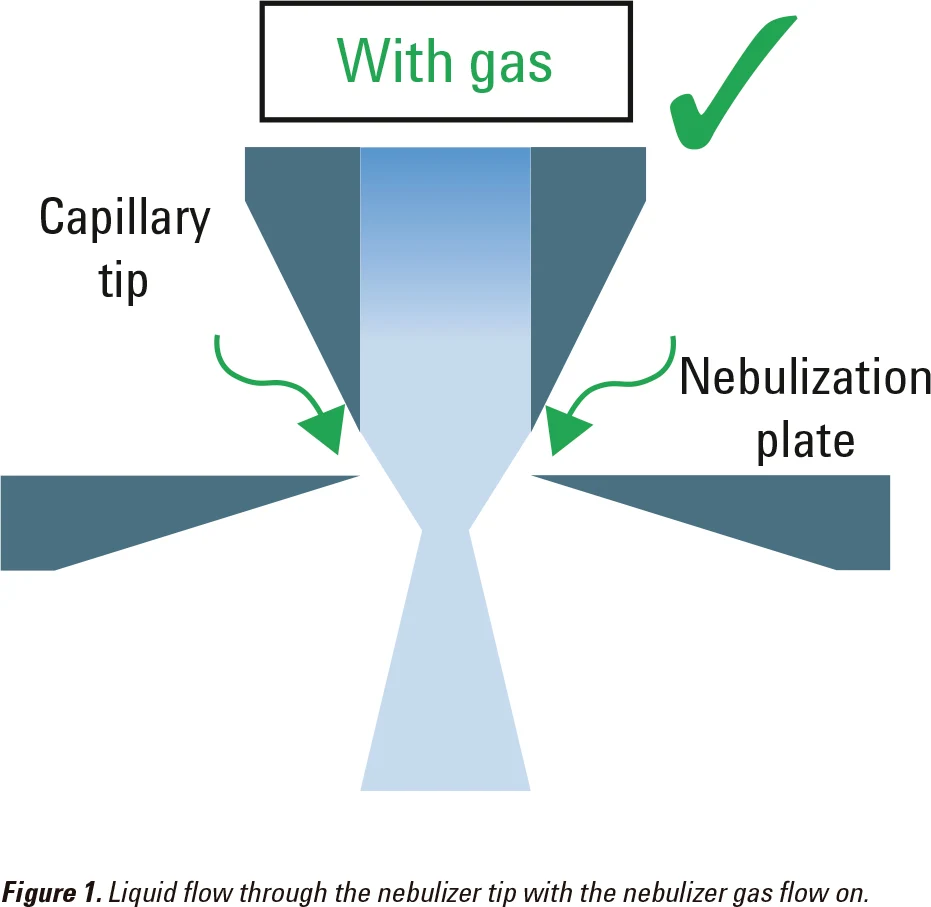
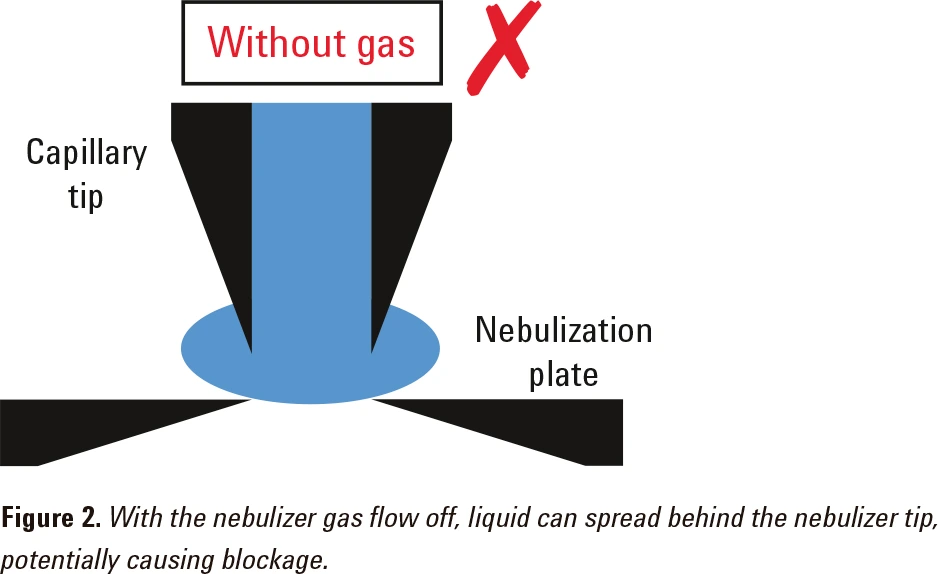
The nebulizer gas flow helps to compress the liquid stream as it flows through the tip of the nebulizer (Figure 1). If there is no nebulizer gas flow, the liquid sample may spread into the gap between the capillary tip and the nebulization plate (Figure 2). This may block the nebulizer.
Use this startup and shutdown procedure to minimize potential blockage:
2.1 Turn on the nebulizer gas flow.
2.2 Start the peristaltic pump to introduce the liquid sample.
2.3 Use the nebulizer normally, rinsing between samples.
2.4 At the end of analysis, always rinse the nebulizer before extinguishing the plasma. Pump a suitable rinse solution for a few minutes. The rinse solution used should have the same matrix as your samples and standards.
2.5 Extinguish the plasma.
2.6 Turn off the nebulizer gas fl ow.
Plasma extinguished during analysis?
If the plasma is unexpectedly extinguished during analysis, rinse the nebulizer with a suitable rinse solution by following the sequence from Steps 2.1 to 2.3. Then resume analysis.
Precautions with high TDS samples
If aspirating samples containing high concentrations of Total Dissolved Solids, always clean the nebulizer by pumping a suitable rinse solution for a few minutes before extinguishing the plasma.
It is also recommended that the portion of the nebulizer which is inside the spray chamber is cleaned regularly to prevent salt deposition at the tip of the nebulizer. If there are visible deposits of material at the tip of the nebulizer, clean the nebulizer before continuing analysis.
Remove large particles to prevent blockage
Reducing dead volume
Always ensure the capillary connector is screwed all the way onto the nebulizer body. This ensures a secure connection and can help to reduce cross contamination. Always connect and disconnect the capillary connector by handling the plastic connector.
Prevent physical damage to the nebulizer
3.1 Do not sonicate the nebulizer in an ultrasonic bath. The vibrations from the bath could damage the nebulizer internally.
3.2 Do not insert foreign objects like cleaning wire into the capillary tip (e.g. to remove blockages). Remove blockage by using the cleaning procedure listed below. If the blockage remains, try using the nebulizer gas fl ow to remove the blockage. Do not exceed a pressure of 700 mbar.
3.3 Handle the sample capillary with care. Never pull on the sample capillary tube. Excessive force applied to the sample capillary can permanently damage the nebulizer or affect its performance.
3.4 Avoid unnecessarily dropping or knocking the nebulizer.
Cleaning the MASSNeb nebulizer
To maintain optimum performance, always rinse the nebulizer after use and clean the nebulizer at least weekly.
Clean the nebulizer by soaking in pure water, dilute detergent solution, or solvent (depending on the application) for several minutes (normally 30 minutes). Rinse thoroughly and dry by passing a stream of fi ltered air, argon, or nitrogen through the tip of the OneNeb. This will help to displace the liquid inside the nebulizer through the gas inlet. Then re-install into the spray chamber. If blockages remain or if suitable performance is not restored after this cleaning process, use the following procedure.
Soak the nebulizer
This requires a standard 5-10 mL plastic syringe and a piece of 4 mm ID plastic tubing 2–3 cm long.
4.1. Prepare a fresh 1M NaOH solution in a 50-mL beaker/container.
4.2. Remove the quick connector for the nebulizer gas supply.
4.3. Connect the syringe to the nebulizer gas inlet tube using the small length of plastic tubing.
4.4. Submerge the tip of the nebulizer in the NaOH solution to a depth of ~2 cm.Use the syringe to draw some of the NaOH solution into the nebulizer. Do not fi ll the nebulizer completely. Ensure the liquid level is below the gas inlet.
4.5. Push the plunger of the syringe to force the NaOH solution out of the nebulizer. Note that some of the NaOH solution enters the sample capillary.Repeat the fi ll/empty procedure at least a couple of times.
4.6. Fill the nebulizer with NaOH solution, disconnect the syringe and allow it to soak, preferably overnight. If the sample capillary siphons the NaOH solution out, it is even better. Remember to place an empty container under the free end of the sample capillary to collect any NaOH solution that is siphoned through the capillary.
Backflush the nebulizer
After soaking, the nebulizer needs to be rinsed by backfl ushing. Backfl ush the nebulizer using the same plastic syringe.
5.1. Connect the syringe to the nebulizer gas inlet and push the plunger to expel any remaining NaOH solution from the nebulizer. Discard this solution.
Disconnect the syringe from the nebulizer.
5.2. Fill the syringe with de-ionized water.
5.3. Connect the syringe to the tip of the nebulizer using the small length of plastic tubing. Slowly depress the plunger of the syringe to force the de-ionized water through the nebulizer tip. Remember to direct the gas inlet tube and the sample capillary to a liquid waste container to collect the waste liquid.
5.4. Repeat steps 5.2 and 5.3 at least twice to thoroughly backfl ush the nebulizer.
5.5. Disconnect the syringe. Dry the nebulizer by directing a stream of fi ltered air, argon or nitrogen through the tip. The flow of gas will displace the liquid inside the nebulizer through the gas inlet. If a gas supply is not available, connect a vacuum to the nebulizer gas inlet using a water jet aspirator or use the syringe to help remove any remaining liquid.
5.6. Reconnect the nebulizer to the instrument and aspirate a rinse solution through the nebulizer for 10-15 minutes.
Let's Make Something Great Together
About
- About
- Products
- Scientific Contribution
- News
Products
Contact
- +34 954 081 214
- info@ingeniatrics.com
- P.I. Parque Plata. C/Camino Mozárabe 41, 41900 Camas, Sevilla.



